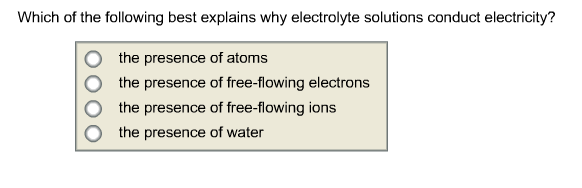
Which Of The Following Best Explains Why Electrolyte Solutions Conduct Electricity?
Which of the following best explains why electrolyte solutions conduct electricity?. A strong electrolyte conducts electricity well. A CO in water B HCl in water C NaCl in water D H2SO4 in water. Electrons are present in electrolyte solutions but they only begin to move when electricity passes through an electrolyte solution.
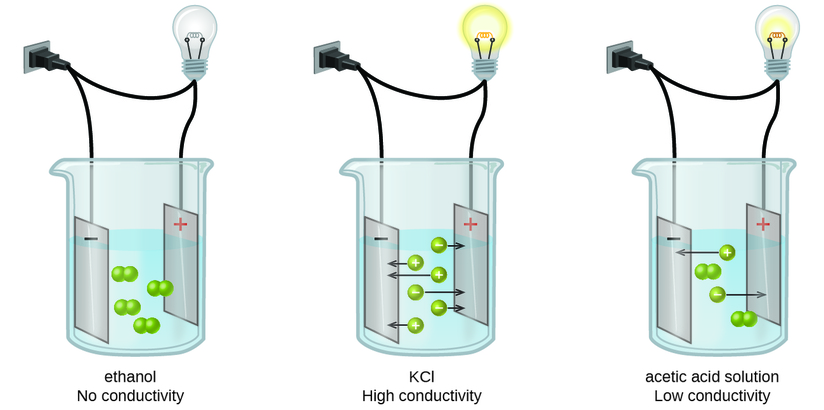
A CO in water. Ethanol CH3CH2OH is a nonelectrolyte. For example NaCl HNO 3 HClO 3 CaCl 2 etc.
All of the bonds in the sucrose molecule are strong covalent bondsTherefore there are no charged particles present to conduct electricity either in the solid state or in solution. Describe some of the major ways in which the conduction of electricity through a solution differs from metallic conduction. An electrolyte is any salt or ionizable molecule that when dissolved in solution will give that solution the ability to conduct electricity.
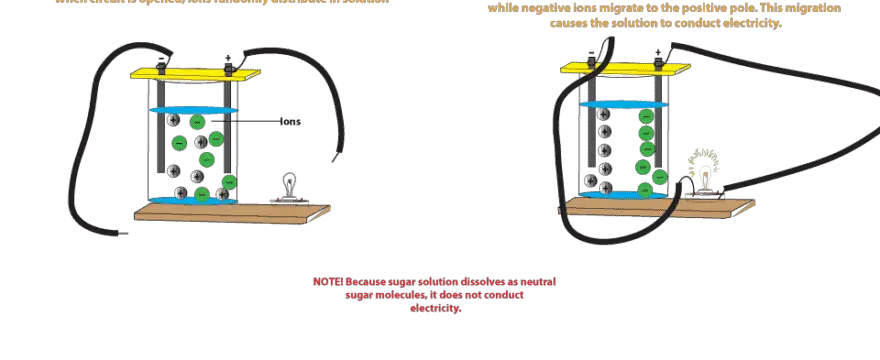
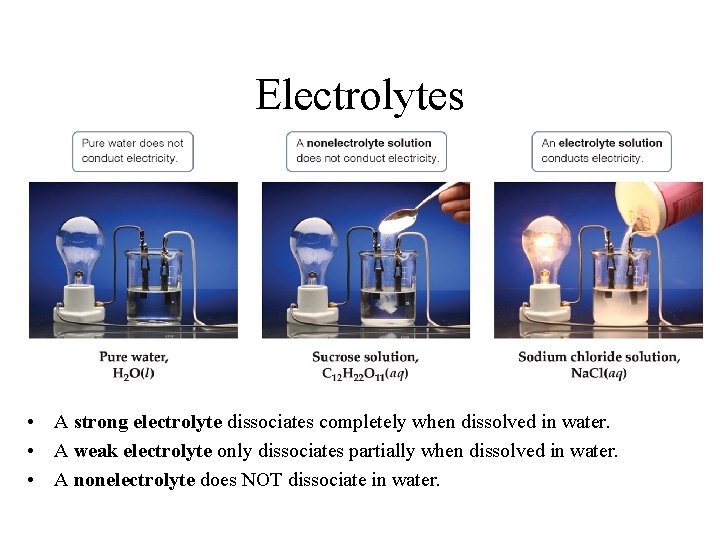
The presence of free-flowing electrons the presence of water the presence of atoms the presence of free-flowing ions. This is because when a salt dissolves its dissociated ions can move freely in solution allowing a charge to flow. Log in Sign up.
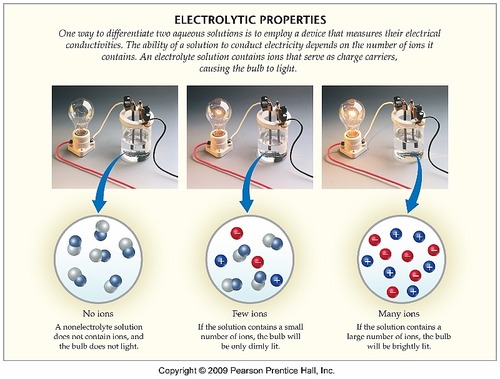
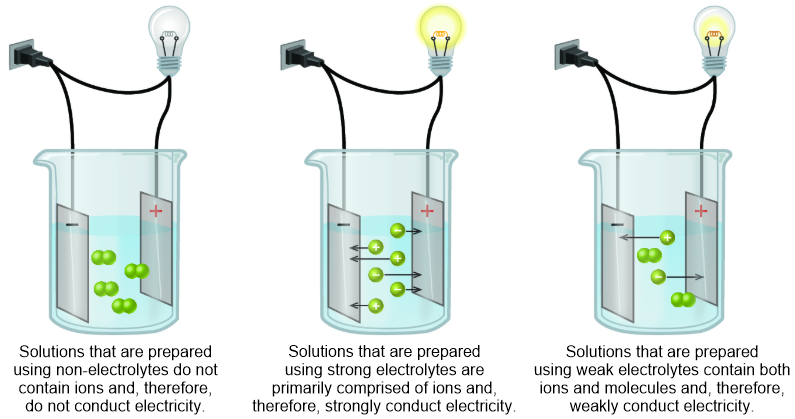
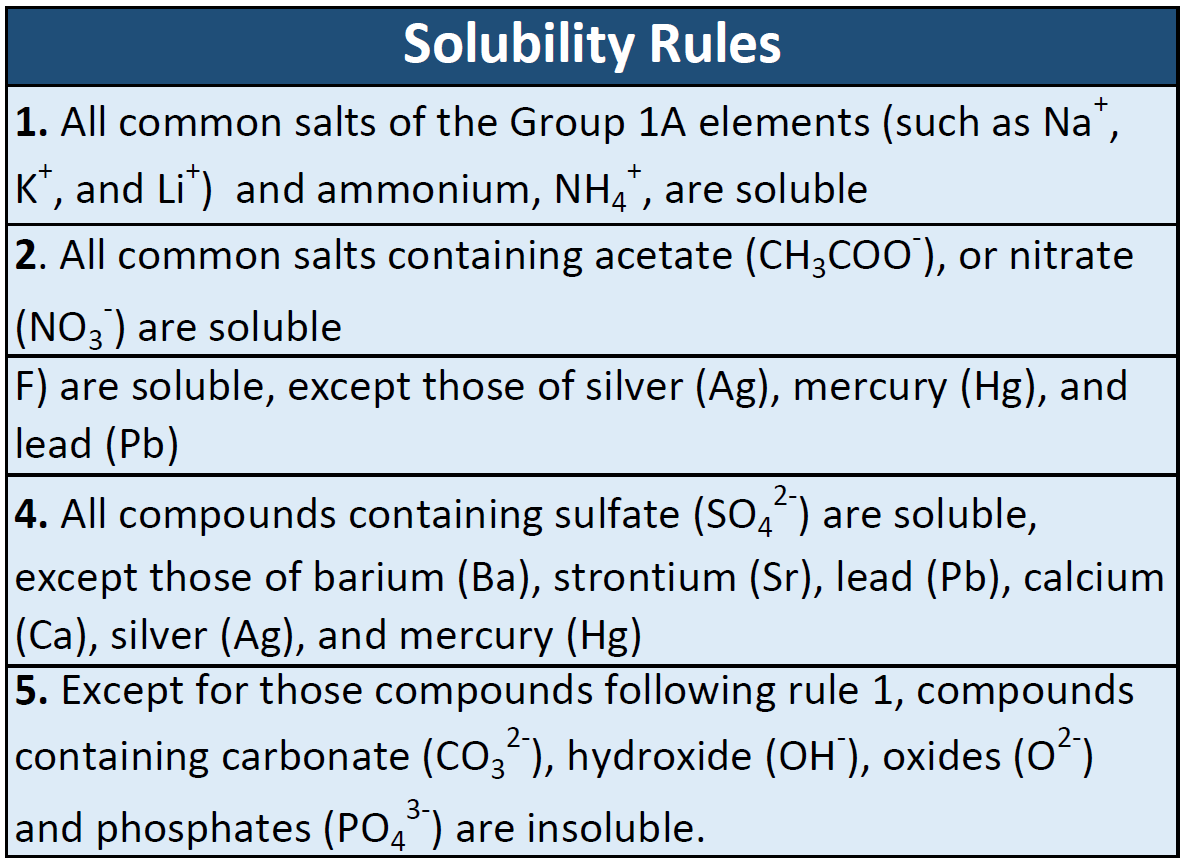
Strong electrolytes completely ionize when dissolved and no neutral molecules are formed in solution. When we speak of ions as conductors of electricity we are not speaking only of electricity we use for lighting our homes or for electrical appliances we find in the home. A sugar solution can conduct electric current better than a salt solutiona.
You can test the solutions by a battery connected to wires and a light bulb into the solution. Non-electrolytes are substances that cannot conduct electricity either in the molten state or in an aqueous solution. Which of the following best explains why electrolyte solutions conduct electricity.
Which of the following best explains why elec. - The Presence Of Free-flowing Electrons - The Presence Of Free-flowing Ions - The Presence Of Water - The Presence Of Atoms This problem has been solved.
Strong electrolytes completely ionize when dissolved and no neutral molecules are formed in solution.
You can test the solutions by a battery connected to wires and a light bulb into the solution. Watch the video solution for the question. Ammonia NH3 is a weak electrolyte. Ethanol CH3CH2OH is a nonelectrolyte. Define resistance resistivity conductance and conductivity. C 2 H 5 OH. The presence of free-flowing electrons the presence of water the presence of atoms the presence of free-flowing ions. D no ions are formed. Substances like sodium chloride which strongly conduct electricity in aqueous solution are called strong electrolytes.
Electrons are present in electrolyte solutions but they only begin to move when electricity passes through an electrolyte solution. For example NaCl HNO 3 HClO 3 CaCl 2 etc. Which of the following solutions would you expect not to conduct electricity. These solutions conduct electricity due to the mobility of the positive and negative ions which are called cations and anions respectively. Watch the video solution for the question. An electrolyte is any salt or ionizable molecule that when dissolved in solution will give that solution the ability to conduct electricity. Non-electrolytes are substances that cannot conduct electricity either in the molten state or in an aqueous solution.






















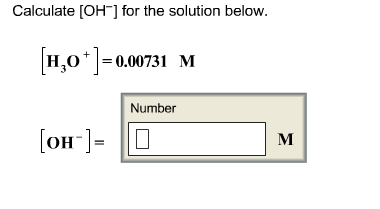














Post a Comment for "Which Of The Following Best Explains Why Electrolyte Solutions Conduct Electricity?"